Login
Login
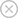
Calcium carbide is a prominent chemical compound known for its various industrial applications and unique properties. Its structural formula, represented as CaC2, highlights the combination of calcium and carbon, comprising one calcium atom and two carbon atoms. This compound plays a crucial role in several sectors, including the production of acetylene gas and the manufacture of various chemical products.
Want more information on structural formula of calcium carbide? Feel free to contact us.
Understanding the basic properties of calcium carbide is essential for those interested in its applications. It is a gray or black solid that reacts vigorously with water, yielding acetylene and calcium hydroxide. The structural formula of calcium carbide signifies a compound that is not only important in chemical processes but also in industries such as welding, metal cutting, and agriculture.
Calcium carbide has remarkable characteristics that contribute to its functionality. For instance, it is highly reactive, especially with moisture, which leads to the release of acetylene gas. This reaction is not only a critical feature but also a central aspect of its utility in generating heat and light in oxy-acetylene flames, commonly used in welding. Furthermore, calcium carbide is relatively stable under dry conditions, which makes it easy to handle during storage and transportation.
The applications of calcium carbide span various fields, making it an indispensable material in many industrial processes. One of the most significant applications is in the production of acetylene, a vital industrial gas used as a fuel for cutting and welding metals. Acetylene's high flame temperature makes it a preferred choice in metalworking, enabling efficient and precise operations.
Beyond its role in acetylene production, calcium carbide is also employed in the manufacturing of chemicals. It serves as a raw material for producing butyne and other organic compounds essential in the chemical industry. The structural formula of calcium carbide further emphasizes its importance as a precursor in various chemical reactions, showcasing its versatility in creating different products.
In agriculture, calcium carbide serves as an effective ripening agent for fruits. By releasing ethylene gas during its reaction with moisture, it accelerates the ripening process, making it valuable for managing the supply chain of perishable goods. Farmers and distributors often utilize calcium carbide to ensure that fruits reach the market at the optimal ripeness, enhancing their marketability.
Safety is a crucial consideration when handling calcium carbide. It must be stored in dry conditions to prevent unwanted reactions with moisture, which could lead to the release of toxic gases. Users are advised to follow stringent safety protocols to minimize risks associated with its use. Proper knowledge of the structural formula of calcium carbide, its reactivity, and its safe handling practices is essential for anyone involved in its application.
Moreover, innovation in the use of calcium carbide continues to evolve. Research and development in related fields are uncovering new applications, enhancing its relevance in modern industrial practices. Companies are exploring how to improve its application efficiency and reduce environmental impacts, aligning with global sustainability goals.
In conclusion, the structural formula of calcium carbide—CaC2—encapsulates the importance of this versatile compound in various industrial sectors. Its ability to produce acetylene, serve as a chemical precursor, and act as a ripening agent in agriculture underscores its multifaceted applications. Understanding its properties, safety measures, and applications provides valuable insights for those engaging with this essential chemical compound. As industries continue to innovate and adapt, calcium carbide remains a fundamental component in advancing technology and efficiency across multiple domains.
Are you interested in learning more about calcium carbide manufacturing process? Contact us today to secure an expert consultation!
25 0 0
Join Us
Comments
All Comments ( 0 )